
Defining the Alternatives
By Dr. Robert Gauthier
Features New Technology ProductionOptions for reducing antibiotic use – what they are, how they work and what the research shows
Chicken production around the world has increased 40 per cent between
1995 and 2005 (+ 12 per cent for Canada) and keeps growing.
Accompanying this growth is an increased demand from consumers
worldwide that the chicken meat and eggs they consume be produced
differently than they have been in the past.
 Chicken production around the world has increased 40 per cent between 1995 and 2005 (+ 12 per cent for Canada) and keeps growing. Accompanying this growth is an increased demand from consumers worldwide that the chicken meat and eggs they consume be produced differently than they have been in the past.
Chicken production around the world has increased 40 per cent between 1995 and 2005 (+ 12 per cent for Canada) and keeps growing. Accompanying this growth is an increased demand from consumers worldwide that the chicken meat and eggs they consume be produced differently than they have been in the past.
Many consumer associations and members of the scientific community around the world strongly contest the use of
antibiotics in animal and poultry production. Hindering this debate are political and commercial interests, as well as national protectionism.
However, the trend towards eliminating antibiotic as growth promotants in livestock and poultry will stay, accelerate and spread to more countries. For many North American integrators, this has become a very lucrative niche market. In the European Union (EU 25), Japan and in the countries producing chickens for those markets, the withdrawal of antibiotic growth promotants (AGPs) is a fact.
Bacterial Resistance
Problems arising from bacterial resistance to antibiotics and the transfer of bacterial resistance to other bacteria (within the same genus or to another genus) is very complex. Another issue is the growing inefficacy of antibiotics as a disease prevention tool or a therapeutic tool.
The antibiotic bacteria resistance problem involves the entire medical profession and the withdrawal of antibiotic growth promoters is only a part of the solution, and is not easy to do to maintain an optimal level of production. It requires management, nutritional and biosecurity changes from the whole chain of production. However, it is possible and many producers worldwide have achieved it successfully and have contributed to the reduction of bacteria resistance to some antibiotics.
REPLACING AGPS
One can only look at what was done with antibiotics over the last 50 years in order to understand the possible mechanisms of poultry growth promotion. These could be described as: Specific enteric disease prevention (e.g., necrotic enteritis), non-specific bacterial growth prevention (dysbacteriosis) and intestinal microflora modulation.
The availability of new biotechnology tools is now aiding in the understanding of the immense complexity of poultry intestinal microflora (which contains more than 600 types of bacteria) and is showing us that the role of each of them is practically unknown.1,2
The identification of a few bacteria types (pathogenic or not) cannot explain the growth promotion effect of antibiotics or similar products. It is well known that AGPs are not working in germ-free chickens and are working much better in poultry grown under sanitary pressure.
Almost anything (age, environmental conditions, feed composition and texture, feed additives, health status, immune status, etc.) can have a strong influence on the composition of the gastrointestinal (GIT) microflora of poultry, but the interpretation of theses changes is still an enormous challenge.3
The role of certain bacteria thought to be beneficial is now challenged because their growth requirements are in direct competition with the host animal for nutrients.
The replacement of AGPs is a difficult and multifaceted challenge that will require an integrated approach and the following citation shows that we might have to revisit our convictions on the subject.
 Intestinal Microflora
Intestinal Microflora
The intestinal microflora is absolutely essential to life, but in the context of poultry production it can reduce animal efficiency through the following mechanisms:5
• Competing with the host for nutrients in the intestinal tract;
• In some circumstances, eliciting an immune response which causes appetite depression and catabolism of muscle protein to fuel this response;
• Disease, particularly necrotic enteritis;
• Lowering digestive efficiency by degrading the digestive enzymes and reducing the absorptive surface areas;
• Increasing the size of the intestinal tract through the production of stimulatory compounds (e.g., polyamines and volatile fatty acids); the net result is an increase in the energy required to maintain the gut, thereby leaving less energy available for productive processes such as muscle deposition.
Viable Alternatives
Today many feed additive suppliers offer a wide range of products that can be used to replace traditional AGPs in swine and poultry. However, even attractive concepts cannot always translate into real efficacy.
To be viable, the products have to be economically affordable, efficacious and technically easy to use on a large scale, safe to the users, the feeds and the target animals.
What follows is a brief review of products or concepts that have potential or that have shown efficacy.
 Organic Acids
Organic Acids
Organic acids have been used successfully in pig production for more than 25 years and continue to be the alternative of choice. Even if much less work has been done in poultry,8 we can confirm today that organic acids are very efficacious, provided their use is adapted to the physiology and anatomy of poultry.
Organic acids (C1-C7) are widely distributed in nature as normal constituents of plants or animal tissues.They are also formed through microbial fermentation of carbohydrates mainly in the large intestine.11 They are also found in their sodium, potassium or calcium form.
Over the years, it was thought that a pH reduction of the GIT content was the mode of action, but research has proven
differently. Research in the food preservation field has brought clear explanations on the mode of action of organic acids on bacteria and numerous trials have shown that the concept works both in pigs and poultry.
• The mode of action of organic acids on bacteria is related to:13
• Undissociated organic acids entering the bacteria cell;
• Bacteria membrane disruption (leakage, transport mechanisms);
• Inhibition of essential metabolic reactions (e.g., decrease of glycolysis);
• Stress on intracellular pH homeostasis (normal bacteria pH is ± neutral);
• Accumulation of toxic anions;
• Energy stress response to restore homeostasis;
• Chelation as permeabilizing agent of outer membrane and zinc binding.
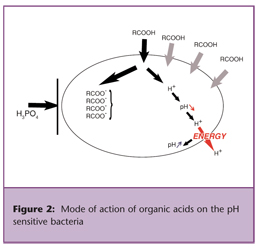
The key basic principle on the mode of action of organic acids on bacteria is that non-dissociated (non-ionized) organic acids can penetrate the bacteria cell wall and disrupt the normal physiology of certain types of bacteria that we call “pH sensitive,” meaning that they cannot tolerate a wide internal and external pH gradient. Among those bacteria are E. coli, Salmonella spp., C. perfringens, Listeria monocytogenes, and Campylobacter spp.
 Upon passive diffusion of organic acids into the bacteria, where the pH is near or above neutrality, the acids will dissociate and lower the bacteria internal pH, leading to situations that will impair or stop the growth of bacteria (refer to Figure 3).
Upon passive diffusion of organic acids into the bacteria, where the pH is near or above neutrality, the acids will dissociate and lower the bacteria internal pH, leading to situations that will impair or stop the growth of bacteria (refer to Figure 3).
On the other hand, the anionic part of the organic acids that cannot escape the bacteria in its dissociated form, will accumulate within the bacteria and disrupt many metabolic functions and lead to osmotic pressure increase, incompatible with the survival of the bacteria.
It has been well demonstrated that the state of the organic acids (undissociated or dissociated) is extremely important to define their capacity to inhibit the growth of bacteria. As a general rule, we need more than 10 to 20 times the level of dissociated acids to reach the same inhibition of bacteria, as compared to undissociated acids14.
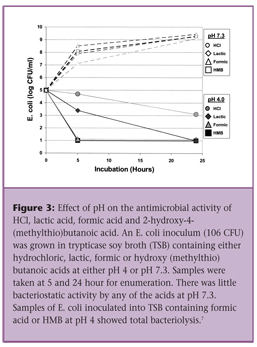
Too often, “in vitro” assays showing the antibacterial capacity of organic acids are done at a low pH, making sure that the acids are not dissociated. At a pH below 3.0-3.5, almost all organic acids are very efficacious in controlling bacteria growth (refer to Figure 4). This does not reflect at all what is happening in the GIT of poultry.
Many authors have studied the effects of organic acids on animals, trying to find an explanation on their mode of action as a growth promoter. Their findings are more related to experiments in pigs but could be partially extrapolated to poultry. Among the explanations, some still believe that the gastrointestinal tract (GIT) content pH change is important even if recent and not so recent publications are showing differently, even with very high acid levels in the feed or water.15,16
 Many are underestimating the capacity of the animal to maintain its GIT environment homeostasis in order to warrant the normal functioning of all digestive functions. Also the strong buffering capacity of the feed prevents any significant GIT pH modification.
Many are underestimating the capacity of the animal to maintain its GIT environment homeostasis in order to warrant the normal functioning of all digestive functions. Also the strong buffering capacity of the feed prevents any significant GIT pH modification.
Logically, organic acids added to feeds should be protected to avoid their dissociation in the crop and in the intestine (high pH segments) and reach far into the GIT, where the bulk of the bacteria population is located (refer to Figure 5).
Other modes of action have been investigated mainly in pigs and cannot apply to poultry: increased digestibility of protein and energy, slower emptying of the stomach (crop – gizzard), modification of the fermentation pattern in the intestine (mostly the large intestine), stimulation of enzyme secretion, modification of the enterocyte development.
All these observations were made in trials using extremely high levels of organic acids incompatible with poultry nutrition.11 Even the modification of the fermentation pattern in the intestine, which is important in pigs, may not apply to poultry because the relative importance of fermentation bacteria in domesticated poultry is much less than in pigs or other mammals.
Also, high intake of organic acids could be harmful to animals. It has been shown that organic acids may lead to a reduction in bone mineral deposition in piglets and many veterinary clinicians are reporting cases of bone decalcification, both in pigs and poultry.17
More likely, the organic acids in poultry might play a direct role on the GIT bacteria population, reducing the level of some pathogenic bacteria (e.g., C. perfringens) and mainly controlling the population of certain types of bacteria that compete with the birds for nutrients.18
Using organic acids in poultry we can expect an improvement in performance similar or better than the antibiotic growth promoters, without the public health concern, as well as a preventive effect on intestinal problems like necrotic enteritis and a reduction of the carrier state for Salmonella spp. and Campylobacter spp.
Essential Oils
Contrary to organic acids, a wealth of research has been done on the use of essential oils, herbs and botanicals in poultry
production, both as a growth promoter or as a disease prevention product.
The scientific and popular press use a lot of different names (plant extracts, phytogenic additives etc.) and to better understand what we are working with, here are some definitions:19
Essential oils: are any of a class of volatile oils obtained from plants, possessing the odour and other characteristic properties of the plant, used chiefly in the manufacture of perfumes, flavours and pharmaceuticals (extracts after hydro-distillation).
Herbs: are flowering plants whose stems above the ground do not become woody and persistent. A plant when valued for its medical properties, flavour, scent or the like.
Botanicals: are drugs made of a plant, as from roots, leaves, barks, etc.
Essential oils or plant extracts can be used as appetite stimulants, aromas, stimulants of saliva production, gastric and pancreatic juices production enhancers and antioxidants. However, there is no clear demonstration of the importance of these factors on chicken performance.
The antibacterial property of essential oils is the most widely studied area, both in human nutrition, food preservation and animal production. Because the control (modulation) of the GIT microflora is the most important aspect in replacing antibiotic growth promoters, I will concentrate on this aspect.
Plants contain hundreds of substances having different properties but essential oils are composed mainly of nine groups (and many sub-groups) of molecules that are of interest. There are many chemical constituents, but no two oils are alike in their structure and effect.
One must distinguish between non-purified plant extracts containing numerous different molecules interacting and pure active compounds, either extracted from plants or synthesized (identical to the natural form). According to the plant chosen, one or more active compounds are dominant and the quantity found will differ according to factors like plant variety, soil, moisture, climate, time of harvest, etc.
It is counterproductive to test every plant that can have interesting properties. Concentrating on the active compounds and selecting the right plants or the right synthetic molecules is easier and will be more acceptable from a regulatory point of view.
Nutritionally, metabolically and toxicologically, we have a clear interest in using levels as low as possible of essential oils in animal nutrition. Essential oils are extremely potent substances; they can lead to feed intake reduction, GIT microflora disturbance, and accumulation in animal tissues and products.
Most essential oils are GRAS (generally recognized as safe) but they must be used cautiously because they can be toxic (contain allergens) and be potent sensitizers, and their odour/taste may contribute to feed refusal.20,21 They are also very volatile and will evaporate rapidly, leading to a large variation in concentration in the finished products. Encapsulation of essential oils could solve the problem.20
The “in vitro” level of most of the essential oils needed to reach a minimal inhibitory concentration (MIC) on various bacteria is high and not applicable in animal nutrition. The number of essential oils really interesting and efficacious as alternatives to antibiotic growth promoters is very limited.
Mode of Action of Essential Oils
It is extremely difficult to generalize on the mode of action of essential oils on bacteria and yeasts because each essential oil has different properties and each type of micro-organism has a different sensitivity. Generally, Gram+ bacteria are considered more sensitive to these oils than Gram- bacteria because of their less complex membrane structure.20
The consensus on the mode of action of essential oils on bacteria is that these compounds influence the biological membranes of bacteria. The cytoplasmic membrane of bacteria has two principal functions:23
A: Barrier function and energy transduction, which allow the membrane to form ion gradients that can be used to drive various processes.
B: Formation of a matrix for membrane-embedded proteins influencing the ATP-synthase complex.
In a study on the mode of action of the essential oil Carvacrol on Bacillus cereus, the researchers showed many aspects of the mode of action of essential oils:23
The inhibition of bacterial growth (Carvacrol is very effective). is a function of concentration, temperature and time;
A sharp reduction of the intracellular ATP pool through a reduction of ATP synthesis and increased hydrolysis, not obviously related to an increase in membrane permeability;
Reduction of the membrane potential (transmembrane electrical potential), which is the driving force for ATP synthesis. The membrane becomes more permeable to protons;
Reduction of the bacteria internal pH with high level of Carvacrol (1mM, pH decreasing from 7.1 to 5.8) related to ion gradient across cell membrane;
Potassium efflux: 1 mM of Carvacrol reduced internal bacteria potassium (K) level from 12 µmol/mg of cell protein to 0.99 µmol in five minutes. K plays a role in the activation of cytoplasmic enzymes, in maintaining osmotic pressure and the regulation of cytoplasmic pH. K efflux is the first indication of membrane damage.
A separate study reported that the mode of action of essential oils is related to an impairment of a variety of enzyme systems, mainly involved in energy production and structural components synthesis.21 This study also explained the mode of action through leakage of ions, ATP, nucleic acids, and amino acids. It also showed that potassium and phosphate ion concentrations are affected at levels below the MIC concentration.
It is interesting to note that most of the levels used “in vitro” to determine MICs are higher than the levels considered acceptable in animal nutrition.
Both organic acids and essential oils (mostly non-purified plant extracts) have been tested as AGP alternatives, alone or in combination. Results are lacking consistency due to the varied composition of products, and varied growing conditions during the trials (environment, feeds, health etc.).
In experiments conducted by Jefo Nutrition Inc. with organic acids, we have experienced very consistent results, both under research station and field conditions. Our rate of positive response exceeded 90 per cent for weight gain and feed conversion, using a blend of protected organic acids.
Not only can protected organic acids act as growth promoter but they can also play a role in the prevention of necrotic enteritis and in the reduction of intestinal Salmonella spp. It appears that the amplitude of the response is often related to the level of contamination or intestinal disease challenge in the flock.
Plants, plant extracts (with their essential oil components) or pure essential oils have been tested quite extensively in many countries with very high result variability. This could be explained by the lack of consistency of the products themselves or simply by the lack of efficacy of certain molecules. As previously outlined, the level of essential oil needed to achieve results is often not compatible with the level allowing the best feed intake.
This leads to using levels that are not efficacious.
Single essential oils, or blends of essential oils, at low levels, are difficult to justify economically and zootechnically.
Synergistic effect
More and more, the concept of combining essential oils and organic acids is proving to be efficacious because there appears to be a synergy between the two concepts.24-26
Both field trials and using a chicken necrotic enteritis challenge model have shown a strong synergy between essential oils and organic acids.
Some studies suggest that the essential oils are damaging the bacteria cell membrane, facilitating the penetration of organic acids into the bacteria cytoplasm.26 But this hypothesis has not yet been demonstrated clearly. Usually the bacteria cell membrane damage leads to an efflux of material from the bacteria, not an influx into the bacteria. However it has been shown that a damaged bacteria cell membrane allowed the uptake of a nuclear fluorescent stain, binding to cellular DNA and RNA.21
The question raised is, are the organic acids behaving like ethidium bromide fluorescent nuclear stain?
Probiotics
Also named “direct-fed microbials” probiotics are mono or mixed cultures of live “protective” microorganisms (defined or not) which affect beneficially indirectly the host by improving the properties of the indigenous microflora.27 Available
probiotics can be classified as colonizing species such as Lactobacillus sp., Enterococcus sp. and Streptococcus sp., which may compete for adhesion sites on the intestinal mucosa (competitive exclusion), or the free flowing “not colonizing” species such as Bacillus sp. and Saccharomyces cerevisiae.
The competitive exclusion probiotics will be administered only once, at hatching time, trying to establish a good microflora that will reduce the colonization of pathogenic bacteria.
The non-colonizing probiotics will be administered on a continuous basis in order to maintain the presence of a minimum quantity of these micro-organisms in the intestinal lumen.
The exact role of probiotics is still questioned and some researchers are even challenging the concept because if a large quantity of bacteria is introduced into the GIT, these bacteria will compete with the bird for nutrients (protein, amino acids, energy).
Not all probiotics are beneficial and some strains have shown negative effects on performance.28
In summary the mode of action relates to: production of specific metabolites (short chain fatty acids, H2O2, intermediary metabolites with antibacterial activity), bacterial interaction (competitive exclusion, colonization resistance ex. Lactobacilli vs pathogens), aggregation and stimulation on the non-specific immune system.
However the results in poultry are very variable and are dependant on the dose and strain and its persistance, the stability during the manufacturing of feeds and in the GIT of birds, the physiological state of the birds and the actual microflora balance.29
Prebiotics
Prebiotics are “non digestible” food ingredients that beneficially affect the host by selectively stimulating the growth of one or a limited number of bacteria in the colon.30 The only practical example of prebiotics are the fructo-oligosaccharides (FOS). This concept is well developed in human nutrition but is not showing interesting results in poultry where the relative importance of the GIT microflora is much less than in mammals.
Some authors have shown that prebiotics can also stimulate the development of pathogenic bacteria.31
The intrinsic composition of FOS and other non-digestible oligosaccharides will show variable results and the obstacles to their use in birds are lack of efficacy, high inclusion rate in the diet and non-economical cost. These products are totally non-nutritive for the birds and have a non-specific effect on the pathogenic microflora.
Is the stimulation of the growth of so-called beneficial bacteria not detrimental to the amino acids and energy availability for the birds?
Yeast Cell Walls
I do not consider yeast cell walls as a prebiotic because they are not stimulating (feeding) the development of bacteria in the GIT.
Two components of the yeast cell walls are of importance; the glucomannan and the ß-1,3/1-6 glucan. Typically, yeast cell walls contain around 50 per cent of polysaccharides on a dry matter basis and of this, around 55 per cent would be mannans and 45 per cent glucans. The problem with this kind of product is that there can be a very wide variation in composition from sources or batches from the same source.
Their more complex modes of action would be related to the blocking of intestinal colonization by pathogenic bacteria via the occupation of adhesion sites (lectins) on the bacteria that have “mannose dependant” adhesion sites. This mode of action is called “Type 1 fimbriae.” However, bacteria have many other adhesion factors towards the intestinal mucosa (such as glycoproteins, glycolipids).
An early study showed that only 46 per cent of E. coli, 48 per cent of Salmonella, 44 per cent of Citrobacter, etc., were positive for agglutination with yeast cell wall (mannose specific lectin).32 Clostridium sp. have no mannose dependent adhesions.
Some authors suggest that there is no relationship between the bacteria agglutination capacity of yeast cell wall components and the real adhesion of certain pathogenic bacteria to the binding sites of the intestinal mucosa.
Another mode of action would be the non-specific stimulation of the GIT immune system via the glucan part of the yeast cell walls. Immune reactivity can also be modulated by nutritional interventions such as alterations in minerals, vitamins, arginine, essential fatty acids and other substances. Bacteria stimulate innate immunity by activating phagocytosis and cytokine synthesis by macrophages. Inflammatory response must be functional without being excessive.
From the review of many publications, yeast cell wall components can have variable results and cannot always stand up to a disease challenge such as necrotic enteritis caused by Clostridium perfringens.34-37 Reasons for that lack of consistency in the results might be the wide quality variation in the products offered to the market and the intrinsic composition of the yeast cell walls not being suited for the effects desired.
Conclusion
The production of poultry without the use of antibiotic growth promoters is already underway in many countries and will keep expanding in the world. The main purpose of this approach is to try to reduce the problem of increasing bacterial resistance towards certain categories of antibiotics.
There are numerous high performing and economical tools to replace antibiotic growth promoters. Research on these is progressing rapidly, and the efficacy of alternatives for poultry will continue to be studied intensively.
REFERENCES
1. Pedroso A.A., J.F.M. Menten, M.R. Lambois, A.M.C. Longo, J.O.B. Sorbara. 2006. Intestinal Bacterial Community and Growth Performance of Chickens Fed Diets Containing Antibiotics. Poultry Science 85:747-752.
2. Lee M. 2002. Microbial Dynamics of the Broiler Intestinal Tract, Abstract, A-6). The Elanco Global Enteric Symposium.
3. Gabriel I., M. Lessire, S. Mallet, J.F. Guillot. 2006. Microflora of the digestive tract: critical factors and consequences for poultry. World’s Poultry Science Journal, Vol. 62, 499-511.
4. Knarreborg A., C. Lauridsen, R.M. Engberg, J.K. Jensen. 2004. Dietary antibiotic growth promoters enhance the bioavailability of a-tocopheryl acetate in broilers through mediations of the lipid absorption processes. J. Nutr. 134:1487-1492.
5. Bedford M. 2000. Removal of antibiotic growth promoters from poultry diets: implications and strategies to minimize subsequent problems. World’s Poultry Science Journal, Vol. 56, 347-365.
6. Gabriel I., S. Mallet, P. Sibille. 2005. La microflore digestive des volailles: facteurs de variation et conséquences pour l’animal. INRA Productions Animales, Vol. 18, No. 5, 309-322.
7. Dibner J.J., P. Butin. 2002. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 11:453-463.
8. Moran E.T. Jr. Comparative nutrition of fowl and swine. The gastrointestinal systems. University of Guelph, Ontario, Canada, 1982.
9. Vanbelle M. 1999. The use of
feed additives in the E.U. Regulations, problems and future. Eastern Nutrition Conference, Animal Nutrition Association of Canada, Niagara Falls, Ontario.
10. Partanen K.H., Z. Mroz. 1999. Organic acids for performance enhancement in pig diets. Nutrition Research Reviews 12:117-145.
11. Foegeding P.M., F.F. Busta. 1991. Chemical food preservation. Disinfection, sterilization & preservation. (S.S. Block editor) Lea Febiger, Philadelphia PA.
12. Brul S., P. Coote. 1999. Preservative agents in foods, mode of action and microbial resistance mechanisms. Intl. J. Food Microbiology 50:1-17.
13. Presser K.A., D.A. Ratkowsky, T. Ross. Modeling the growth rate of Escherichia coli as a function of pH and lactic acid concentration. Applied & Environmental Microbiology, June 1997, Vol. 63, No. 6, 2335-2360.
14. Cheveerach P., D.A. Keuzenkamp, L.J.A. Lipman, F. Van Knapen. 2004. Effect of organic acids in drinking water for young broilers on Campylobacter infection, volatile fatty acid production, gut microflora and histological changes. Poultry Science 83:330-334.
15. Waldroup A., S. Kaniawati, A. Mauromoustakos. 1995. Performance characteristics and microbiological aspects of broilers fed diets supplemented with organic acids. Journal of Food Protection 58:482-489.
16. Biagi G., A. Piva, T.D. Hill, D.K. Schneider, T. Cranshaw. 2003. Bone mineral content gain is reduced in weaned pigs fed diets with low-buffer capacity and organic acids. J. Anim. Sci. vol 81, suppl 1/J. Dairy Sci. vol. 86, suppl 1. Poster M98.
17. Lee M.D. 2005. Molecular basis for AGP effects in animals, p. 37-38. Antimicrobial Growth Promoters: Worldwide Ban on the Horizon. Noordwijk aan Zee, The Netherlands, Jan 31-Feb 1.
18. Webster’s Encyclopedic Unabridged Dictionary of the English Language, 1989.
19. Lis-Balchin M. 2003. Feed additives as alternatives to antibiotic growth promoters: botanicals. Proceedings of the 9th International Symposium on Digestive Physiology in Pigs, Banff AB, Canada. University of Alberta, publisher. Vol. 1, p. 333-352.
20. Lambert R.J.W., P.N. Skandamis, P.J. Coote, G.-J.E. Nychas. 2001. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. Journal of Applied Microbiology, 91:453-462.
21. Friedman M., P.R. Henika, R.E. Mandrell. 2002. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes and Salmonella enterica. Journal of Food Protection, 65:1545-1560.
22. Ultee A., E.P.W. Kets, E.J. Smid. 1999. Mechanisms of action of carvacrol in the food-borne pathogen Bacillus cereus. Applied and Environmental Microbiology 65;4606-4610.
23. Van Wesel A.A.M., H.B. Perdok, D.J. Langhout. 2004. Phasing out antimicrobial growth promoters. II Congresso Latino Americano De Suinicultura, Foz do Iguaçu, Brasil, 20-22 Outubro, p. 141-144.
24. van Dam J.T.P., M.A.M. Vente-Spreeuwenberg, H.P.T. Kleuskens. 2005. Combination of medium chain fatty acid and organic acids provides a cost-effective alternative to AGP in pig nutrition, P5. Antimicrobial Growth Promoters: Worldwide Ban on the Horizon. Noordwijk aan Zee, The Netherlands, Jan 31-Feb 1.
25. van Kol E.M.R. 2005. Organic acids and essential oils in AGP free diets, P7. Antimicrobial Growth Promoters: Worldwide Ban on the Horizon. Noordwijk aan Zee, The Netherlands, Jan 31-Feb 1.
26. Huyghebaert G. 2005. Alternatives for antibiotics in poultry. Proceedings of the 3rd Mid-Atlantic Nutrition Conference, University of Maryland, College Park, MD 20742.
27. Bosi P., L. Casini, P. Trevisi, S. De Filippi, M. Mazzoni. 2005. New Topics and Perplexities on the Use of Probiotics in Animal Feeding. Proceedings of the 1st International Seminar on Probiotics in Animal Nutrition. Rome Italy.
28. O’Dea E.E., G.M. Fasenko, G.E. Allison, D.R. Korver, G.W. Tannock, L.L. Guan. 2006. Investigating the Effects of Commercial Probiotics on Broiler Chick Quality and Production Efficiency. Poultry Science 85:1855-1863.
29. Gibson G.R., M.B. Roberfroid. 1995. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutrition 125:1401-1412.
30. Monsanthin R. cited by P. Best. 2000. Starter pig feeds: Oligosaccharides. Do these feed sugars assist the right bacteria. Feed International, February, pp. 24-26.
31. Mirelman D., G. Altmann, Y Eshdat. 1980. Screening of Bacterial Isolates for Mannose-Specific Lectin Activity by Agglutination of Yeasts. Journal of Clinical Microbiology. Vol. 11, No. 4, 328-331.
32. Huff G.R., W.E. Huff, N.C. Rath, G. Tellez. 2006. Limited Treatment with b-1,3/1,6-Glucan Improves Production Values of Broiler Chickens Challenged with Escherichia coli. Poultry Science 85:613-618.
33. Spring P., C. Wenk, K.A. Dawson, K.E. Newman. 2000. The Effect of Dietary Mannanoligosaccharides on Cecal Parameters and Concentration of Enteric Bacteria in the Ceca of Salmonella-Challenged Broiler Chicks. Poultry Science 79:205-211.
34. Ferket P., C.W. Parks, J.L. Grimes. Mannan oligosaccharides versus antibiotics for turkeys. Alltech Annual Symposium.
35. Hofacre C.L., T. Beacorn, S. Collet, G. Mathis. 2003. Using Competitive Exclusion, Mannan-Oligosaccharides and other Intestinal Products to Control Necrotic Enteritis. J. Appl. Poult. Res. 12:60-64.
36. Parks C.W., J.L. Grimes, P.R. Ferket. 2005. Effects of Virginiamycin and a Mannanologosacchaide-Virginiamycin Shuttle Program on the
Growth and Performance of Large White Female Turkeys. Poultry Science 84:1967-1973. n
Print this page