
ILT, an emerging disease
By By N.C. KIRKPATRICK H.P. BLACKER S. RUBITE D. O’ROURKE and A.H. NOORMOHAMMADI
Features New Technology ProductionWEB EXCLUSIVE
ILT, an emerging disease
In 2007 and 2008, a series of Infectious Laryngotracheitis virus (ILTV) outbreaks were recorded in commercial layer and broiler flocks, mostly occurring in Victoria, Australia.
In 2007 and 2008, a series of Infectious Laryngotracheitis virus (ILTV) outbreaks were recorded in commercial layer and broiler flocks, mostly occurring in Victoria, Australia. The control of these outbreaks was complicated by the shortage of the key commercially available Australian vaccines (SA2 and A20). Previous studies in our laboratory, using polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analyses, have identified five different genotypes of ILTV. Most of the ILTV isolates from these recent outbreaks belonged to the same ILTV genotype. A novel ILTV genotype, that was distinct from all recent and historical isolates in Australia, was also discovered in several flocks of a single broiler company. Australian vaccine strains SA2 and A20 were not found responsible for any of the outbreaks investigated in this study.
The threat
ILT has been identified in many countries worldwide and remains a threat to the intensive poultry industry. It is caused by an alphaherpes virus, involved in respiratory disease of varying severity and reduced egg production. Outbreaks of mild to moderate forms of ILT are not uncommon in commercial layer flocks worldwide, while sporadic outbreaks of ILT in broiler flocks has also been recognized in recent years as an emerging problem in several countries, including Australia.
Field evidence indicates that many ILT outbreaks may have been due to lack of uniform flock immunity and the transmission of vaccine strain from vaccinated to unvaccinated birds (Andreasen et al., 1989; Hilbink et al., 1987). Additionally, it is speculated that some live attenuated vaccine strains of ILTV may occasionally revert to parental-type virulence, causing sporadic ILT outbreaks (Guy et al., 1991). The live attenuated vaccines SA2 and A20 (Fort Dodge, Australia, Pty. Ltd.) and Nobilis ILT (Intervet Pty. Ltd.) are used to immunize chickens against ILT in Australia. The vaccine strain SA2 originated from an Australian field isolate and was attenuated through sequential passages in chicken embryo (Purcell and Surman, 1974), while strain A20 originated from SA2 through further passages in chicken embryonic cell culture to lessen residual virulence (Bagust, pers. comm.)
The Nobilis vaccine consists of a live attenuated Serva strain of ILTV and has only recently been introduced into the Australian market. While the A20 vaccine is considered relatively safe and can be used in very young chickens, the SA2 and Nobilis ILT vaccines are less attenuated and are only recommended for use in adult birds or as a booster vaccine after A20 administration. ILTV strains may vary in their virulence in chickens, chicken embryo, and cell culture.
METHODS
The commercial ILTV vaccine strains SA2, A20 and Nobilis ILT and several recent ILTV isolates were used in this study. All field isolates were isolated from the upper respiratory tract and conjunctiva of infected birds during outbreaks of ILT in Australia. All virus isolates were propagated on chicken embryo kidney (CEK) cells using standard techniques (Tripathy and Hanson, 1980). Scrapings of infected tissue from the trachea and conjunctiva were diluted in cell culture medium and inoculated onto CEK cells. Infected CEK cells were frozen at -80C then thawed, centrifuged at 400 x g for five minutes and the supernatant was removed and used for DNA extraction. DNA was extracted from CEK cells supernatant, commercial vaccines, or directly from swabs taken from infected trachea using a method described previously (Skyes et al., 1997, in Kirkpatrick et al., 2006). Viral DNA was extracted from tracheal swabs, a 50µL volume of reconstituted commercial vaccines or infected CEK cell supernatant, as described previously (Kirkpatrick et al., 2006).
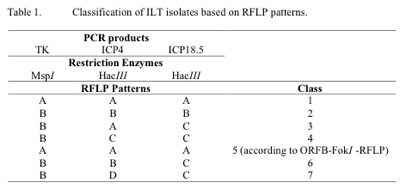
Primers for ILTV genes TK, ICP4 and ICP 18.5 that were used in this study, PCR reactions and subsequent RFLP analyses were each previously described by Kirkpatrick et al., 2006. Consequently, restriction endonuclease enzymes MspI and HaeIII were used for restriction digestion of the PCR products of the genes TK, ICP4 and ICP 18.5, respectively (Table 1). Restriction DNA fragments on polyacrylamide gels were visualized by staining with GelRed (Biotium, Hayward, CA) and subjected to digital imaging using the Kodak GelDoc system. Classification of the ILTV isolates was carried out using RFLP patterns generated from genes TK, ICP4 and ICP18.5 as described by Kirkpatrick et al., 2006.
RESULTS
A total number of 26 ILTV isolates were examined in this study, of which all except one were from Victoria. Analysis of the ILTV isolates resulted in two new classes of ILTV, 6 and 7, not reported in our previous study (table 1). As seen in table 1, class 5 is only distinguishable from class 1 when an additional PCR-RFLP procedure is followed.
|
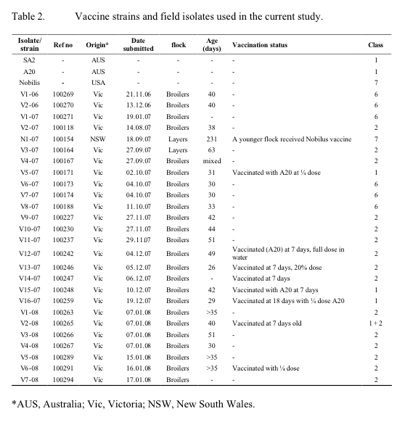
All submissions were made from commercial farms. Only two isolates were from layer birds, the remainder was from broilers. Except where indicated in table 2, all isolates were from unvaccinated birds and were involved in increased mortalities at the farms. Only mild clinical symptoms (conjunctivitis and rhinitis) and not increased mortality were associated with isolates from vaccinated birds. Most of the isolates examined in this study were found to belong to class two, while a number of isolates from a discrete region in Victoria (V1 -06, V2-06, V1-07, V6-07, V7 -07, and V8-07) were found to belong to class six, which is distinct from all previously described classes. Also, one isolate (N1-07) was found to be distinct from all previous ILTV isolates and to be indistinguishable from the Nobilis ILT vaccine strain (class seven). Investigation into the history of this isolate indicated an increased mortality and upper respiratory symptoms in unvaccinated birds that had been placed in the same shed as birds vaccinated two weeks earlier with the Nobilis ILT vaccine.
 |
DISCUSSION
In outbreaks where rapid differentiation of ILTV isolates from vaccine strains is critical, the TK PCR-RFLP can easily be used as a preliminary screening test. RFLP of other genes can then be used to further identify the isolate for more in -depth epidemiological studies. The majority of the recent ILTV isolates isolated from outbreaks in commercial flocks were found to be distinct from the vaccine strains SA2 and A20. The exceptions were the isolates V5-07, V15 -07, V16-07 and V5-08, each of which had identical RFLP patterns to the vaccine strains. All these isolates, however, were from birds vaccinated with the A20 vaccine strain and unlike other outbreaks, were not involved with increased mortalities. There is therefore no suggestion that the vaccine strain underwent an increase in virulence (Guy et al. 1991).
The isolates found to belong to class six were obtained from farms belonging to a single poultry company in the same Victorian locality. The ILTV class two isolates in this study were from two different commercial broiler companies and a single layer company. Further investigation on the source of outbreaks related to this class of ILTV did not reveal a link to the possible transmission of the virus from one company to another although it was found that all affected farms received feed from the same feed mill company. Further investigation into the involvement of air/wind and insects may be necessary to fully understand the route of transmission of ILTV in the recent outbreaks.
ACKNOWLEDGMENTS
Funding for this project was provided by the Australian CRC. The authors would like to thank Dr. Peter Scott for scientific input, Tony Belfiore, Peter Cowling, and Kylie Hewson for technical support, the Victorian Department of Primary Industries (DPI) for the supply of some of the ILTV isolates, and field veterinarians for submission of clinical cases.
1 Faculty of Veterinary Science, The University of Melbourne, Werribee, Victoria, 3030
2 Baiada Poultry, Laverton North, Victoria, 3026
REFERENCES:
Andreasen, J. R., Jr., Glisson, J. R., Goodwin, M.A., Resurreccion, R.S., Villegas, P. and Brown, J. (1989) Avian Diseases. 33:524 ñ530. Guy, J.S., Barnes, H.J. and Smith, L. (1991) Avian Diseases. 35:348 ñ355.
Hilbink, F.W., Oei, H.L. and van Roozelaar, D.J. (1987) Veterinary Quarterly. 9:215ñ 225.
Kirkpatrick, N.C., Mahmoudian, A., O’Rourke, D. and Noormohammadi, A.H. (2006) Avian Diseases. 50:28-34.
Purcell, D.A. and Surman, P.G. (1974) Australian Veterinary Journal. 50:419 ñ420.
Sykes, J.E., Browning, G.F., Anderson, G., Studdert, V.P. and Smith, H.V. (1997) Archives of Virology. 142:65 ñ74.
Tripathy, D.N. and Hanson, L.E. (1980) Isolation and identification of avian pathogens. Hitchner, S.B., Domermuth, C.H., Purchase, H.G., and Williams, J.E. eds. American Association of Avian Pathologists, College Station, Texas. pp. 88 ñ90.
Print this page