
Robustness of Laying Hens
By BY L. STAR H.K. PARMENTIER J.J. VAN DER POEL AND B. KEMP wageningen University the netherlands
Features New Technology ProductionIs it all about genes, environment or early-life
Is it all about genes, environment or early-life experiences?

|
| Genetic background plays a role in how hens cope with stress. |
Summary
Immune competence and physiological parameters (production and endocrine) during various environmental stress conditions were studied in purebred layer lines at various ages.
Our purpose was to establish the contribution of genetic background, environment and early-life experiences to the so-called “robustness.” Next to egg production, levels of innate and specific immune competence depended on genotype. Within breeds, however, innate immune competence was related with survival. Comparable response patterns to climatic and immune stress were found within breeds, but breeds differed in response levels towards these stressors. The response levels could not be influenced by early-life experiences. Our data suggest that robustness mainly depends on the capacity to respond to stressors within a genetic background, and that the maintenance of different fitness strategies within a selected purebred may favour coping with different environments on the long term.
Introduction
There are a limited number of inter-nationally operating poultry breeding companies that provide laying hens worldwide. For these companies, it is favourable to have animals that can function under a wide variety of environmental conditions. In addition, robustness is a term that is rapidly becoming a main interest in animal production. 1
Robustness can be defined as an animal under a normal physical condition that has the potential to keep functioning and take short periods to recover under varying environmental conditions. Robustness of an animal probably depends on genetic potential, environmental influences, and early-life experiences, and robustness can be evaluated in terms of physiological, behavioural, and immunological traits (Figure 1).
Three experiments were performed to establish the importance of genetic potential, environmental influences, and early-life experiences on the robustness of laying hens. Results of these experiments will be described briefly. Thereafter, a discussion will follow on robustness in relation to genetic potential, environmental influences, and early-life experience. The discussion will end with a suggestion of which parameter or trait is of interest for implementation into a breeding goal for robustness.
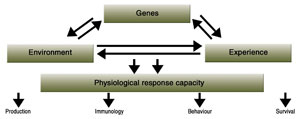
|
| Figure 1. Robustness of an animal is influenced by genetic background, environment and early-life experiences, where production, survival, behaviour, immune and physiological parameters can be used as read-out for robustness. |
Results
2a. Genetic potential
A population of 1,063 laying hens was used to establish natural antibody (NAb) levels and haemolytic complement activity. Within this population 12 purebred layer lines (Hendrix Genetics, Boxmeer, the Netherlands) could be distinguished: six White Leghorn lines (W1, WA, WB, WC, WD, and WF) and six Rhode Island Red lines (B1, B2, B3, BA, BB and BE).
Differences between layer lines in levels of natural humoral immune competence were established, but most importantly, the data suggests, regardless of line, levels of Nab were related to the probability of surviving the
laying period.2
2b. Environmental influence
From the former experiment (2a), four of the 12 lines (WA, WB, WF, and B1) were selected, based on a profile of high or low natural immune competence and a high or low survival rate. These lines were exposed to the following environmental stressors: heat (climatic stress), lipopolysaccharide (LPS, hygienic stress), or combined exposure to heat and LPS.3-5
Hens were able to cope with single or combined heat stress and LPS challenge. The lines had similar response patterns, but differed in response levels, suggesting that some lines were better able to adapt to stressors than others. Lipopolysaccharide and heat stress initiated sequential responses over time, with an earlier effect of short-term LPS exposure (within the first and second week) and a later effect of long-term heat exposure (within the second and third week), indicating that heat stress and LPS challenge acted like two independent stressors.
2c. Early-life experience
From the former experiment (2b), one line (B1) was selected. Hens of this line were not able to maintain a high hen-day egg production during heat stress. Effects of early-life experience with heat stress on adaptability to the same stressor in later-life were studied (Star et al., submitted). The data suggest that early-life heat stress exposure did not affect adaptability of laying hens to heat stress in later-life.
Discussion
Comparable response patterns to climatic and immune stress were found within breeds, but breeds differed in response levels towards these stressors. Line B1 had, for instance, a strong reduction in feed intake, body weight, and hen-day egg production during heat stress compared to lines WA, WB, and WF. The response levels of line B1 could not be influenced by early-life experiences. Hester et al. concluded from their studies that, from criteria used to evaluate stress (e.g., physiological and immuno-logical parameters), egg production and mortality provided the best evidence for adaptability to stress.6-8 Our data also suggest that some lines are better able to cope with environmental stressors than other lines based on egg production and mortality. Line B1 had a high mortality rate under commercial circumstances and showed a decline in hen-day egg production by exposure to high temperatures.2 Line WA had a decline in hen-day egg production at the end of the laying period
(60 to 69 wk of age) under commercial circumstances (unpublished data) and had more problems with keeping up production under heat stress than the other White Leghorn lines. Line WB and WF were able to maintain a high hen-day egg production under heat stress. However, line WF was a better survivor under commercial circumstances than line WB (Star et al., 2007a), which makes line WF a more “robust” line. Our data suggest that robustness mainly depends on the capacity to respond to stressors within a genetic background, and that the maintenance of different fitness strategies within a selected purebred may favour coping with different environments on the long term.
The conclusion about robustness as discussed above is, however, based on line difference. For the final goal of these experiments it was important to find traits on individual level that could be implemented into a breeding goal. In future livestock systems it is necessary that breeding goals should not only be defined in terms of production, but that they should also include traits related to animal health and welfare. Therefore, the findings described in the first experiment (2a) are of interest and probably most important for robustness. Regardless of line, low levels of NAb binding to keyhole limpet haemocyanin (KLH) were detected in chickens that did not survive the laying period (Figure 2a).
 |
Recently, we have established a similar relation between NAb binding to KLH in a crossbred line (Figure 2b). In the same crossbred population we have estimated a heritability of NAb binding to KLH of 0.23, indicating that selection for NAb levels is possible. In practical – commercial – context, however, selection for this “robustness” trait must be in balance with selection for production traits.
Acknowledgments
This research is part of a joint project of Institut de Selection Animale, a Hendrix
Genetics Company, and Wageningen University on “The genetics of robustness in laying hens” which is financially supported by SenterNovem. n
References
- Knap, P.W. (2005). Australian Journal of Experimental Agriculture, 45 :763 -773.
- Star, L., Frankena, K., Kemp, B., Nieuwland, M.G.B. and Parmentier, H.K. (2007a). Poultry Science, 86:1090 -1099.
- Star, L., Nieuwland, M.G.B., Kemp, B. and Parmentier, H.K. (2007b). Poultry Science, 86:1894 -1903.
- Star, L., Van den Anker, I., Kemp, B. and Parmentier, H.K. (in press). Poultry Science.
- Star, L., Decuypere, E., Parmentier, H.K. and Kemp, B. (in press). Poultry Science.
- Hester, P.Y., Muir, W.M. and Craig, J.V. (1996a). Poultry Science, 75 :1315 -1320.
- Hester, P.Y., Muir, W.M., Craig, J.V. and Albright, J.L. (1996b). Poultry Science, 75 :1295 -1307.
- Hester, P.Y., Muir, W.M., Craig, J.V. and Albright, J.L. (1996c). Poultry Science, 75 :1308 -1314.
Print this page