
Tracking HPAI in the wild
By Treena Hein
Features Disease watch Migration MattersMonitoring HPAI in the environment as best we can is critical, in order to detect and monitor which strains are appearing and how they may change over time. That said, here's an update on research and surveillance happening in North America.
 U.S. scientists have published the first study in North America involving the tracking of a wild bird – a lesser scaup – known to have HPAI.
Photo: Jeffrey Sullivan
U.S. scientists have published the first study in North America involving the tracking of a wild bird – a lesser scaup – known to have HPAI.
Photo: Jeffrey Sullivan As everyone in the Canadian poultry industry knows, it’s critical for commercial poultry producers and backyard flock owners to follow all biosecurity procedures to their flocks from the highly-pathogenic avian influenza strain (HPAI) that arrived in North America about a year ago – in addition to other strains that may exist.
On that note, monitoring the virus in the environment as best we can is critical, in order to detect and monitor which strains are appearing and how they may change over time. Different strains can vary in virulence, contagiousness and other characteristics.
In Ontario, surveillance continues, using samples taken from dead wildlife that the public have found and brought to the attention of the Canadian Wildlife Health Cooperative (CWHC). “We are currently seeing cases of HPAI in many species of wild birds and mammals,” reports Dr. Brian Stevens, a veterinarian and CWHC wildlife pathologist.
“The most common birds affected recently have been Canada geese and turkey vultures,” he says. “We have also had one fox and one skunk die from this disease this fall. This is very similar to what we saw in the spring, with the only difference being that we saw the disease commonly in bald eagles as well. We have diagnosed it in many species of wild birds, but most are in the general categories of waterfowl, shorebirds, raptors and corvids.”
U.S. insights
Meanwhile, scientists at the U.S. Geological Survey have published the first study in North America involving the tracking of a wild bird known to have HPAI. This team is collaborating on other AI research projects with colleagues at the University of Delaware, Maryland Department of Natural Resources, U.S. Fish and Wildlife Service, University of Georgia and Ducks Unlimited.
This study came about by chance. The scientists were not aware that the bird – a lesser scaup duck found in the Chesapeake Bay area of Maryland – was infected with HPAI when they released it back into the wild. They had captured it for another research project and had observed no signs of infection. However, results a week later from a swab test taken as a part of the research came back positive.
In knowing this bird’s movements and comparing it to 24 other birds in the study that were not infected, the research team noted it had moved shorter distances in similar timeframes. That is, the infected bird was, as would be expected, less active compared to non-infected birds.
However as expected, during the time the team tracked this group of birds, the non-infected bird was in close contact with non-infected birds and there were opportunities for potential transmission. Transmission of HPAI or AI can happen from bird to bird or from bird to environment to bird (typically through fecal-contaminated water).
When asked if other bird species are likely similar in that infected individuals move around less than non-infected, team member Dr. Diann Prosser says that’s difficult to answer.
“There is still relatively little data on which to base these assumptions, and many aspects of AI pathobiology vary by species,” she says. “Thus, we should be cautious when attempting to extrapolate based on limited data.
“We are actively working to obtain additional disease samples from other telemetry projects that include different waterfowl species in an effort to grow our dataset.”
The extent of the presence of this strain in the environment is not discernable by the research team currently. “It’s not known where the virus is in the environment, nor the survivability of this strain of viral material once it’s shed into the environment,” Prosser says. “However, understanding how the wild bird reservoirs move once they become infected is important for modeling disease spread following both initial introductions but also across seasons, and the annual cycle of wild waterfowl.”
However, as readers of this magazine will recall, environmental surveillance of AI has been happening in B.C. for several years.
Wetland sediment surveillance
AI surveillance involving collecting samples from wild birds killed by hunters or found dead is difficult. Live capture and release on a large scale is not feasible due to costs, stress on the birds, ecological disruption and so on.

A team of B.C. scientists attained a 20 per cent rate of virus detection by testing wetland sediments.
Photo: Michelle Coomb
In 2015, after the major AI outbreak in B.C., the provincial government sought the development of a novel AI surveillance approach. Scientists at the B.C. Centre for Disease Control (BCCDC), CWHC and other partners began a pilot study to test wetland sediments (where wild bird feces accumulates) for virus presence.
Note that the methods being used did not and do not distinguish between live (potentially infective virus in the environment) and dead virus but look at sediment as an alternative or complementary method for detecting viruses circulating in wild birds instead of looking at sediment as part of the bird-environment-bird transmission cycle.
The results of the pilot tests were very promising, with a 20 per cent rate of virus detection compared to less than a one per cent rate of detection through wild bird swab sampling. This is not surprising, because in each wetland sediment sample, the feces of potentially several birds can be present, making the chances of getting a viral RNA sample much higher.
In the pilot study, the team used sampling technology from a B.C. firm called Fusion Genomics. However, because the firm’s probes are proprietary and limit the ability to add new probes as new AIV variants emerge, the team has developed its own probes.
The plan, says PhD student Kevin Kuchinski (UBC and BCCDC), was to make an open-source probe system and release a research manual for others around the world to use, covering from how to best sample to how to make the probes to how to best do data analysis. There is great interest from various teams in the EU and the U.S. about this system.
This was supposed to be achieved by 2021, but with the pandemic having gotten in the way, the system should be ready for other research teams in the coming year.
“This new probe technique directly characterizes the virus as it exists in the sediment and bird swabs,” Kuchinski says. “Using conventional sampling and analysis, which involves culturing of the virus, viruses can mutate.”
Current testing
The team generally does not do sampling in the summer because warmer temperatures and stronger sunlight speed up the destruction of viral RNA. So, the last time they did surveillance sampling was last fall/winter, before the arrival of this HPAI had made it to B.C. after landing on Canada’s Atlantic coast.
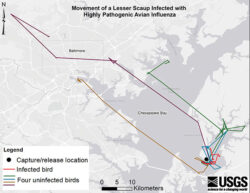
This illustration shows the movement of a lesser scaup infected with highly patogenic avian influenza that researchers monitored.
Photo: United States Geological Survey
The team has started up sampling again now, as always in the Fraser Valley area, in several wetlands that span an area that’s about 125 km long. Sediment samples are also coming in from agencies in other parts of Canada.
“The surveillance this winter will give us more information about what’s going on in the environment around chicken farms,” says PhD student Michelle Coombe (UBC and B.C. Ministry of Ag).
“This may assist the chicken boards and public health agencies in setting biosecurity alerts for the poultry industry in lower mainland B.C. and beyond. There’s obviously a high biosecurity risk right now from this virus, but it’s important to be very aware of what strains are in the environment that are circulating and to better understand the ecology of the virus as well.”
Kuchinski says, “I think one of the big questions is whether this variant lineage will be endemic here, as it has become endemic in Europe and Asia. We hope to see how widespread the lineage is and how it may have mutated.”
He adds that the arrival of this strain in Atlantic Canada last winter has really highlighted the fact that surveillance needs to be everywhere to be more effective.
“The system is getting to the point where we can roll it out in the near future,” he says. “This will hopefully really help poultry farmers prevent outbreaks because they have that early warning. It’s hard to get that if you’re blind to what strains are out there, where they are moving and how they may be mutating. Wetlands are the key.”
Print this page